- A multicenter study - a study that is carried out in a large number of specialized clinical centers;
- Randomized - a study in which patients are distributed into groups randomly;
- Double-blind, placebo-controlled – a study in which neither the study physician nor the patient knows whether the patient is receiving
the study drug or a placebo.
Study of Memo in chronic
brain ischemia
Mexidol® and Mexidol® Forte 250 preparations showed a high degree of efficiency and safety in the international multicenter randomized double blind, a placebo-controlled study of evaluating the effectiveness and safety of consistent therapy of patients with chronic brain ischemia-memo [1].


The purpose of the study was to study the effectiveness
and safety of consistent therapy with Mexidol®
Parentutral (intravenously) and Mexidol® Fort 250 orally
(using tablets) in patients with chronic
brain ischemia.
- A multicenter study - a study that is carried out in a large number of specialized clinical centers;
- Randomized - a study in which patients are distributed into groups randomly;
- Double-blind, placebo-controlled – a study in which neither the study physician nor the patient knows whether the patient is receiving
the study drug or a placebo.

The purpose of the study was to study the effectiveness
and safety of consistent therapy with Mexidol®
Parentutral (intravenously) and Mexidol® Fort 250 orally
(using tablets) in patients with chronic
brain ischemia.
Research design
The study of Memo was attended by 318 patients aged 40 to 90 years with chronic brain ischemia of 15 clinical centers of various regions of Russia and Uzbekistan. Patients were randomized in 2 groups.
-
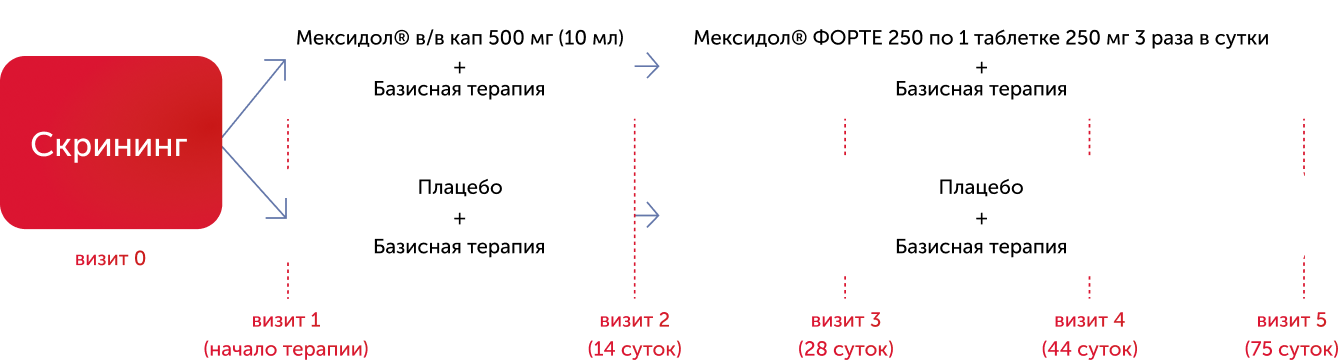
The study scheme included:
- visit 0 (no more than 7 days) - screening period;
- visit 1 - the beginning of parenteral therapy;
- visit 2 - completion of parenteral therapy;
- visit 3 - a telephone survey of the patient;
- visit 4-completion of the 1st month of tablet therapy;
- Visit 5 - a final visit for 60 days of tablet therapy.
Patients of the 1st group (159 people) received a long sequential therapy with Mexidol® according to the scheme: Mexidol® in a dose of 500 mg per day intravenously 14 days with the subsequent transition to the tablet form of Mexidol® Forte 250, 1 tablet 250 mg three times per three times aid three times Day against the background of basic therapy for 60 days. The second group of patients (159 people), together with basic therapy, received a placebo according to a similar scheme.
Research design memo
15 clinical centers (Russian Federation + Republic of Uzbekistan)
Population - patients with chemicals (318 people were randomized)
Age of 40 - 90 years. Average age 60.4 +-9.45 years
The total duration of therapy is 75 days (2.5 months)
(Parenteral - 14 days, oral - 2 months)
Efficiency criteria
As the primary criterion of effectiveness, the average value of the change in the point on the Montreal scale of cognitive function assessment (MOCA) was estimated at the stage of completion by the patient compared to the initial level.
As secondary criteria for effectiveness, the dynamics assessment used:
- On the Montreal scale of cognitive assessment (MOCA) in all visits,
- according to the dialing test of digital characters (DSST),
- on the subjective scale of asthenia assessment (MFI-20),
- On the Bek anxiety scale,
- on the scale of vegetative status (Wane questionnaire),
- On the scale of balance and walking Tinetti,
- according to the questionnaire for assessing the quality of life SF-36,
- On the scale of the general clinical impression (CGI)
The safety of therapy was evaluated on the basis of the presence and severity of the undesirable phenomena recorded during the study.
Results
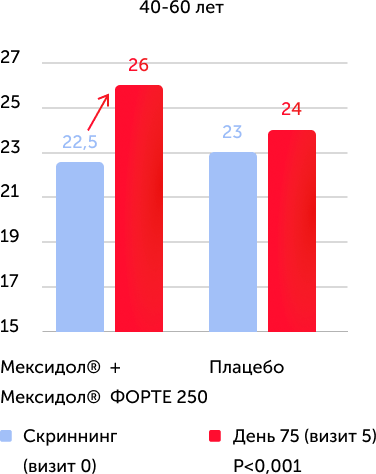
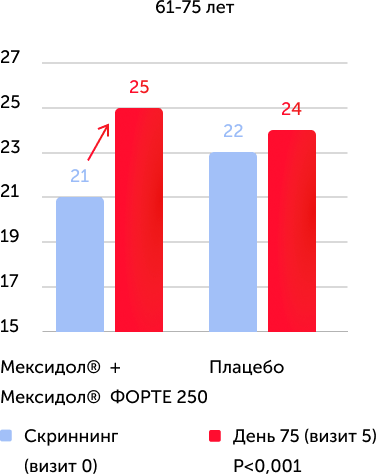
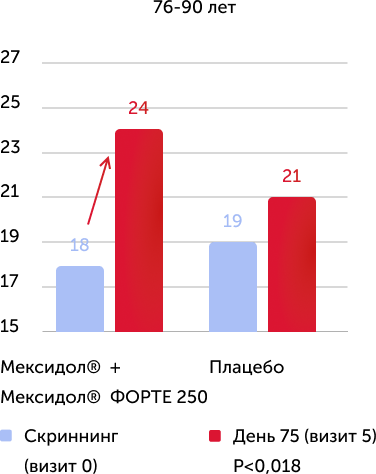
When analyzing the results of the primary criterion for the effectiveness in the Mexol® group, the improvement and normalization of cognitive functions to the end of therapy (75th day) was demonstrated: +4.2 points on the Mosa scale, which is significantly more than in the comparison group: +2, 2 points.
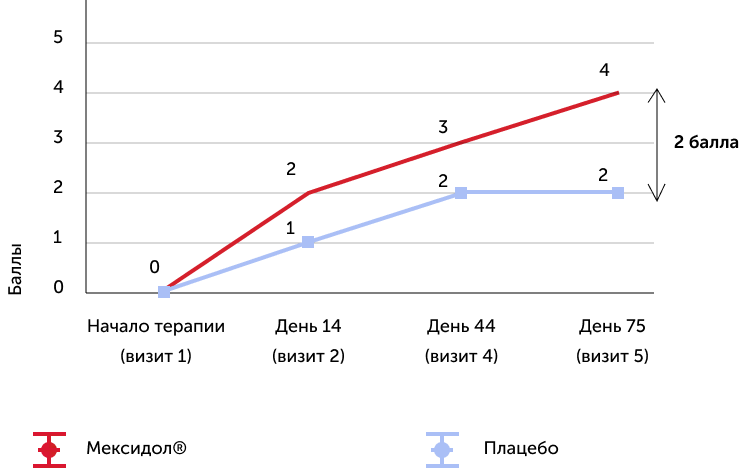
The dynamics of changes in the average score on the Mosa scale
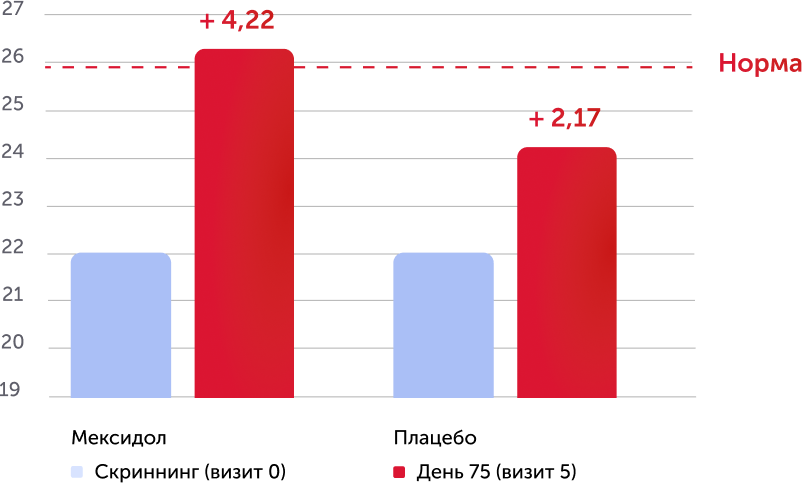
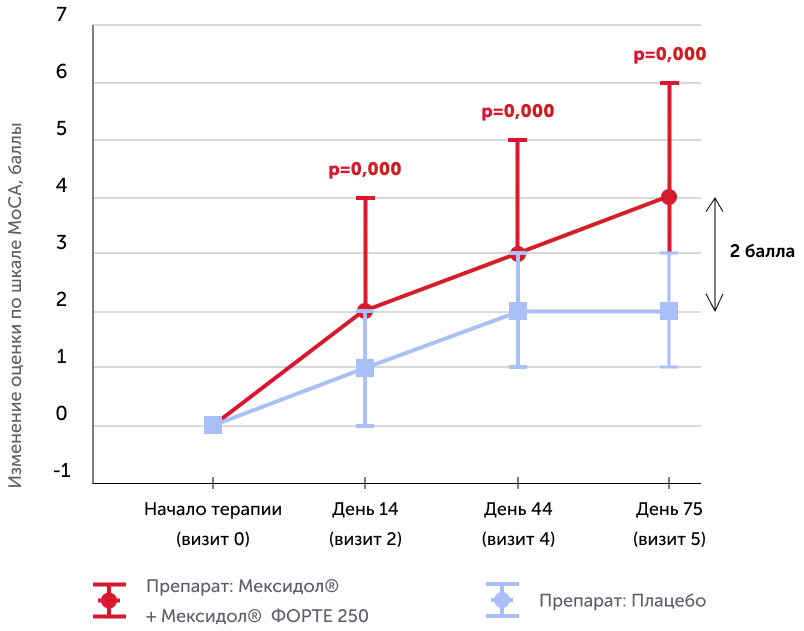
The assessment of the results of secondary effectiveness criteria showed that the total average score on the Mosa scale in the main group reached the norm - 26.22 points, and in the placebo group remained at the level of cognitive violations - 24.17. The differences were reliable, starting from 2 visits (the completion of parenteral therapy), a positive dynamics increased against a long -term consistent therapy. Thus, the results obtained indicate the high effectiveness of the drug Mexidol® in the chronic ischem of the brain in relation to the main symptom - cognitive disorders.
Dynamics on the Mosa scale
Medians of the absolute dynamics of points in the scale in the study groups, the 25th and 75th percentiles, and the R-meaning of the Mann-Uinty criterion are also given
Digital characters replacement test, dsst
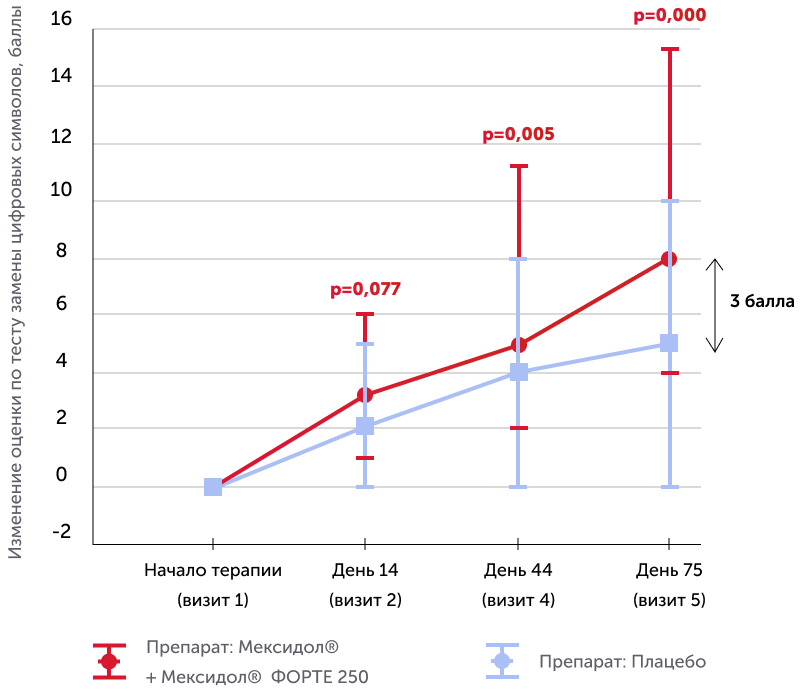
Medians of the absolute dynamics of points in the scale in the study groups, the 25th and 75th percentiles, and the R-meaning of the Mann-Uinty criterion are also given
The study showed a significant increase in the average score for the change in digital characters (+8 points in the group with the drug Mexidol® compared to +5 points in the placebo group).
Subjective scale for assessment of asthenia MFI-20
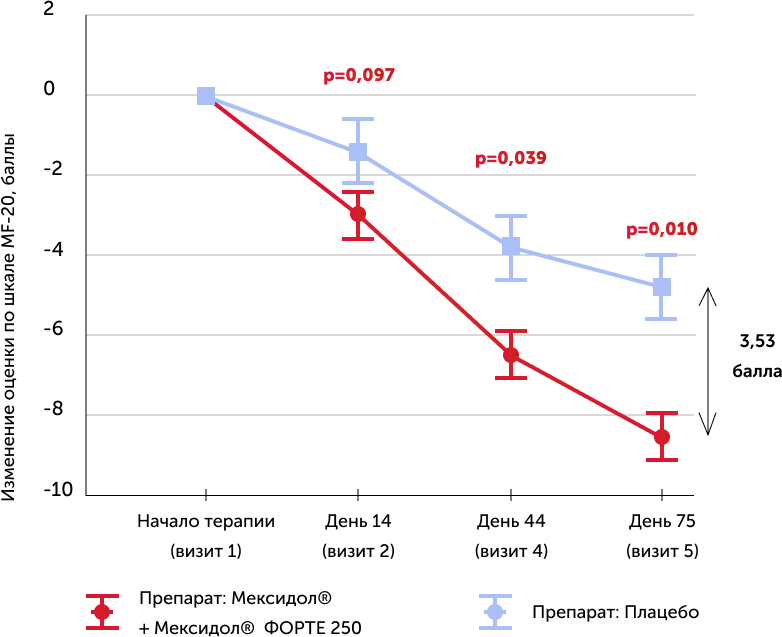
The average values of the absolute dynamics of points in the scale in the study groups and 95% trust intervals for medium, as well as the R-meaning of the criterion of the T-CRITENT T-CRITENT
When evaluating the severity of asthenic syndrome by the end of therapy, a reliable decrease in asthenia in patients of the main group (Mexidol®) was revealed: -8.3 points on the MFI -20 and -4.8 point in the comparison group (placebo).
Bek anxiety scale
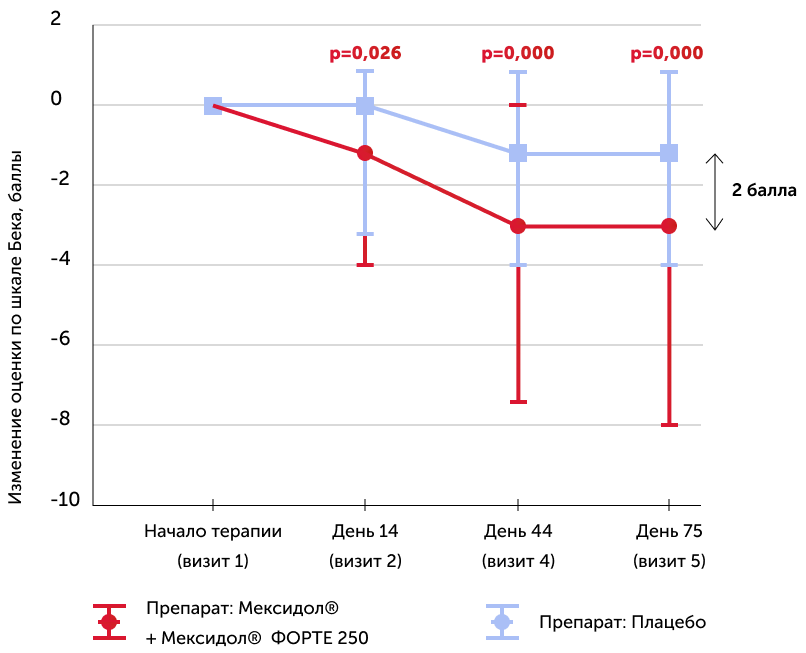
Medians of the absolute dynamics of points in the scale in the study groups, the 25th and 75th percentiles, and the R-meaning of the Mann-Uinty criterion are also given
The results of the examination on Bek’s anxiety showed a reliable decrease in anxiety level in patients of the 1st group (Mexidol®) by the end of therapy (75th day): -3 points, and in the 2nd group (placebo) -1 points.
Wane vegetative status scale
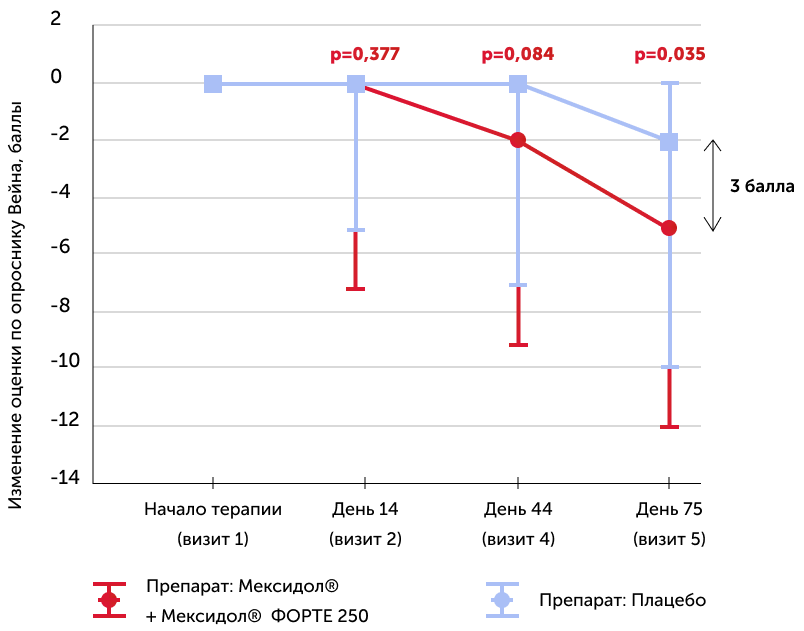
Medians of the absolute dynamics of points in the scale in the study groups, the 25th and 75th percentiles, and the R-meaning of the Mann-Uinty criterion are also given
The study of the vegetative status in patients of both groups showed a statistically significant decrease in the manifestations of autonomic dysfunction in patients of the group with Mexidol® (-5 points) compared to the placebo group (-2 points) by the end of therapy (75th day).
Tinetti
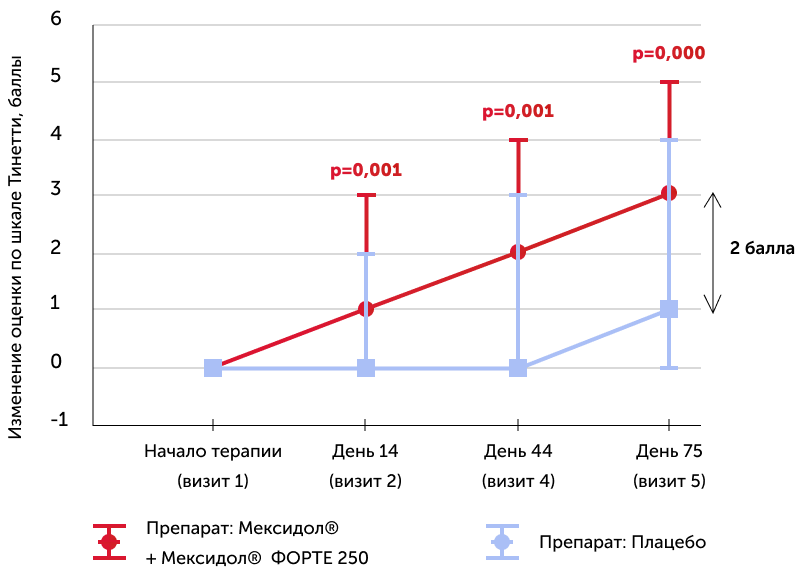
Medians of the absolute dynamics of points in the scale in the study groups, the 25th and 75th percentiles, and the R-meaning of the Mann-Uinty criterion are also given
When evaluating the equilibrium and walking of Tinetti, an improvement in the indicators was established in the group of the host Mexidol® +3 points, and in the placebo group +1 points, and statistically significant differences between groups were determined immediately after the end of injections of the drug Mexidol®.
Assessment of the quality of life of the patient SF-36
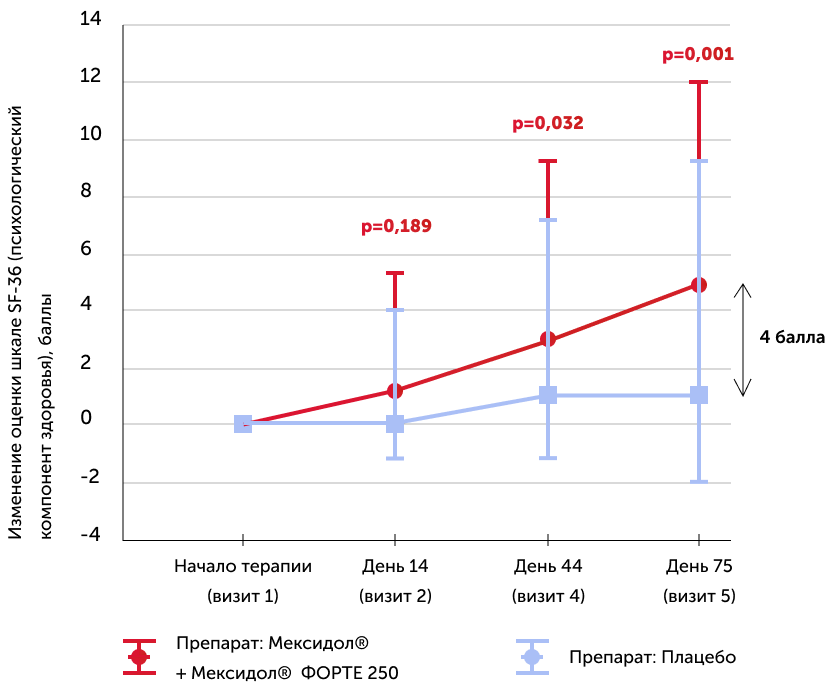
Medians of the absolute dynamics of points in the scale in the study groups, the 25th and 75th percentiles, and the R-meaning of the Mann-Uinty criterion are also given
A significant improvement in the quality of life in patients of the group 1 (Mexol®) was noted by the end of therapy (75th day), as patients (+5 points for the SF-36 VS +1 point in the placebo group), and treating doctors who noted “Strong or noticeable improvement” on the scale of the general clinical impression of CGI more than 53% of Mexidol® patients and 15% in the placebo group, and 48% of the placebo group on this scale “The condition has not changed/worsened”. Taking the drug Mexidol® in chronic brain ischemia significantly influenced the quality of life.
Thus, it can be said that prolonged sequential therapy with Mexidol® and Mexidol® Forte 250 contributed to a reliable reduction in the level of cognitive impairment, autonomic manifestations, equilibrium disorders, walking, a decrease in asthenia, a decrease in anxiety, and improving the quality of life and well -being. Positive dynamics was increasing in nature when evaluating all the above scales, tests and questionnaires, reaching the maximum of effectiveness to the end of a long consistent therapy (i.e. on the 75th day), which once again proves the importance of a full course of therapy.
Conclusions
The results of the study convincingly indicate the high clinical efficiency of prolonged sequential therapy with Mexidol® and Mexidol® Forter Cerebus Cerebus, including in all age groups, which was expressed in the reliable regression of such important manifestations of chronic brain ischemic as cognitive, emotional, vegetative and motor Violations.
The simultaneous significant regression of all the main clinical manifestations of chronic brain ischemia is an important argument in favor of the fact that therapy with Mexidol® affects the pathogenetic links of chronic vascular brain damage, not limited to symptomatic improvement.
Safety
All unwanted phenomena registered in this study had an easy and moderate degree of severity, the differences between the groups were statistically insignificant. The results of laboratory analyzes and physics examination also testified to the lack of significant differences between the compared groups.
List of literature
- Fedin A.I., Zakharov V.V., Tanashyan M.M., Chukanova E.I., Majidova E.N., Shchepanevich L.A., Ostroumova O.D. The results of an international multicenter randomized double-blind-controlled study of the assessment of the effectiveness and safety of consistent therapy of patients with chronic brain ischemia drugs Mexidol and Mexidol Forte 250 (study of Memo). Journal of neurology and psychiatry named after S.S. Korsakova. 2021; 121 (11): 7–16.
Fedin Ai, Zakharov VV, Tanashyan MM, Chukanova EI, Madzhidova En, Shchepankevich La, Ostroumova OD. REZULITY MEZHDUNARODNOGO MNOGOTROVOGO RANDOMIZIROVANOGO DVOINOGO SLETSEBO-KONTROLIREMOGO ISSLEDOVANIA OTSENKI EFFECTIVNOSTII I BEZOPASNOSTI Sledovatel'noi TERAPII PATSIENTOV S KHRONICOI ISHEMIEI MOZGA PREPARATAMI MEKSIDOL I MEKSIDOL FORTE 250 (IssLEDOVANIE MEMO) [Results an Internamed Multicenter, Randomiz Ed, Double-Blind, Placebo- Controlled Study Assessing the Efficacy and Safety of Serape with Mexidol and Mexidol Forte 250 in Patients with Chronic ISChemia (Memo)]. ZH NEVROL PSICHIATR IM SS Korsakova. 2021; 121 (11): 7-16. Russian. DOI: 10.17116/JNEVro2021121117. PMID: 34932280. - Zakharov V.V., Tkacheva O.N., Mkhitaryan E.A., Fedin A.I. The effectiveness of Mexidol in patients of different age groups with chronic brain ischemia with cognitive disorders (the results of subanalysis of the international multicenter randomized double-blind placboo-controlled study of memes). Journal of neurology and psychiatry named after S.S. Korsakova. 2022; 122 (11 Issue 2): 73–80.
Zakharov VV, Tkacheva On, Mkhitaryan Ea, Fedin Ai. Effektivnost 'MEKSIDOLA U PATSIENTOV RAZNYKH Vozrastnykh Gronicheskoi ISHEMIEI Golovnogo Mozga s kognitivnymi narusheniyami Dunarodnogo mnogotSentrovogo randomizirovannogo dvoinogo Slepogo Platsebo-Kontroliruemo IssleDovaniya Memo) [Efficacy of Mexidol in Patents of Emia and Cognitive Impairment of Different Age Groups ( Results of Sub-ANALYASIS OF THE INTERNATIONAL MULTICENTER, RANDOMIZED, DOBLE-BLIND, PLACEBO-CONTROLLED SEXEPYOPY in PATIENTS Mo)]. ZH NEVROL PSICHIATR IM SS Korsakova. 2022; 122 (11. VYP. 2): 73-80. Russian. DOI: 10.17116/JNEVro202212211273. PMID: 36412160. - Zakharov V.V., Ostroumova O.D., Kochetkov A.I., Klepikova M.V., Fedin A.I. International multicenteric randomized double-tied placebo-controlled study of evaluating the effectiveness and safety of sequential therapy of patients with chronic brain ischemia drugs Mexidol and Mexidol Forte 250: Subanalysis results in patients with arterial hypertension. Therapy. 2023; 9 (1): 145–159.
THE INFORMATION IS INTENDED FOR HEALTHCARE AND PHARMACEUTICAL PROFESSIONALS. THIS INFORMATION IS NOT INTENDED AS A SUBSTITUTE FOR MEDICAL ADVICE.
Source of photos and images Shutterstock.com