- Многоцентровое исследование — исследование, которое проведено в большом количестве специализированных клинических центров.
- Рандомизированное — исследование, в котором происходит распределение пациентов на группы случайным образом.
- Двойное слепое плацебо-контролируемое — исследование, в котором ни врач-исследователь, ни пациент не знают, что получает пациент: исследуемый препарат или плацебо.
Исследование МЕГА
при синдроме дефицита внимания и гиперактивности
у детей
Препарат Мексидол® показал высокую степень эффективности и безопасности
в лечении синдрома дефицита внимания и гиперактивности (СДВГ) у детей
в ходе многоцентрового двойного слепого рандомизированного
плацебо-контролируемого клинического исследования (МЕГА) [1].

Доказана эффективность схемы терапии препаратом Мексидол®
при СДВГ – по 1 таблетке 125 мг 2 раза в день в течение 1,5 месяцев.
Повторные курсы могут быть рекомендованы через 2—3 месяца [2].
режимов дозирования препарата Мексидол® таблетки, покрытые пленочной
оболочкой, 125 мг (ООО «НПК „ФАРМАСОФТ“, Россия), по сравнению с плацебо
у детей с СДВГ в возрасте от 6 до 12 лет.
- Многоцентровое исследование — исследование, которое проведено в большом количестве специализированных клинических центров.
- Рандомизированное — исследование, в котором происходит распределение пациентов на группы случайным образом.
- Двойное слепое плацебо-контролируемое — исследование, в котором ни врач-исследователь, ни пациент не знают, что получает пациент: исследуемый препарат или плацебо.
Дизайн исследования
Исследование МЕГА проводилось в трех параллельных группах, в нем принимали участие 333 ребенка в возрасте от 6 до 12 лет с подтвержденным диагнозом СДВГ, установленным в соответствии с критериями МКБ-10 и DSM-5, все пациенты были рандомизированы в 3 равные по численности группы.
- Пациенты 1-й группы получали Мексидол® 125 мг 2 раза в день;
- Пациенты 2-й группы получали Мексидол® 125 мг 1 раз в день + плацебо;
- Пациенты 3-й группы получали только плацебо.
Продолжительность лечения во всех группах составила 42 дня (рис. 1).
Дизайн исследования МЕГА
14 клинических центров
Многоцентровое, двойное слепое, рандомизированное, плацебо-контролируемое в трех параллельных группах клиническое исследование по оценке эффективности и безопасности препарата Мексидол® в лечении синдрома дефицита внимания с гиперактивносью (СДВГ) у детей (МЕГА)
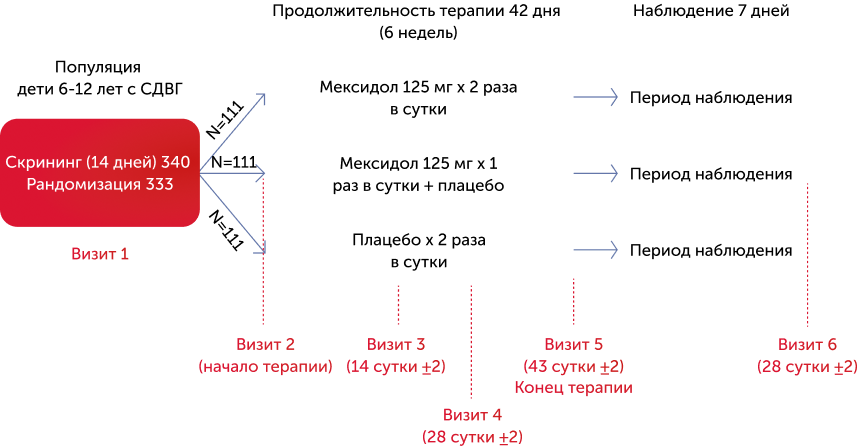
Рисунок 1. Дизайн исследования МЕГА

Критерии эффективности
В качестве первичного критерия эффективности изучалось среднее изменение суммы общего балла по субшкалам «невнимательность», «гиперактивность/импульсивность» шкалы SNAP-IV (опросник, состоящий из 43 вопросов, позволяющих в баллах оценить степень нарушения внимания, гиперактивности и импульсивности) через 6 недель терапии по сравнению с исходным уровнем [3].
К вторичным критериям эффективности были отнесены средние изменения через 6 недель терапии по сравнению с исходным уровнем:
- по субшкале SNAP-IV «невнимательность»,
- по субшкале SNAP-IV «гиперактивность/импульсивность»,
- по субшкале SNAP-IV «оппозиционно-вызывающее расстройство поведения»,
- индекса Коннерса (определяет рейтинг внешнего наблюдателя и самоотчета для оценки дефицита внимания и гиперактивности),
- общей оценки симптомов СДВГ по шкале ADHD Rating Scale-IV (опросник, состоящий из 18 критериев СДВГ),
- оценки по шкале общего клинического впечатления степени тяжести СДВГ (CGI-ADHD-S) [4],
- оценки по шкале общего клинического впечатления — улучшение (CGI- I) [5],
- общей оценки по шкале детской тревожности (SCAS) [6].
Результаты
При анализе результатов первичного критерия эффективности было установлено, что по окончании 6 недель терапии были получены статистически значимые изменения суммы общего балла по субшкалам «невнимательность», «гиперактивность/импульсивность» шкалы SNAP-IV. Наиболее выраженная динамика отмечалась в группе Мексидол® 125 мг 2 раза в день (-29% от исходного уровня). Между всеми группами имели место статистически значимые различия (рис. 2).
Изменение в % значений среднего изменения суммы общего балла по субшкалам “невнимательность”, ”гиперактивность/импульсивность” SNAP-IV через 6 недель терапии
Первичный критерий эффективности
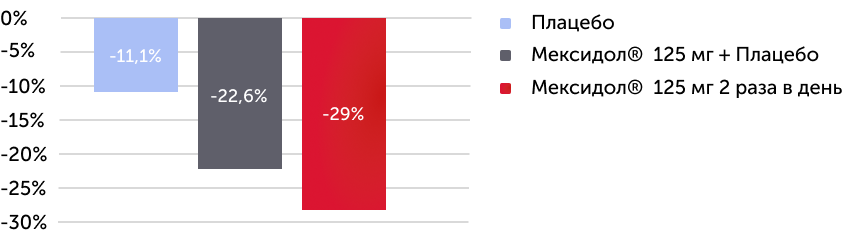
Статистически значимые изменения оценок по субшкалам “невнимательность”, ”гиперактивность/импульсивность” SNAP-IV через 6 недель терапии были выявлены во всех трех группах пациентов. (p < 0,000001)
В группе Мексидол® 125 мг 2 раза в день отмечался 29% регресс основных проявлений СДВГ
Рисунок 2. Результаты первичного критерия эффективности – динамика изменения суммы общего балла по субшкалам «невнимательность», «гиперактивность/импульсивность» шкалы SNAP-IV через 6 недель терапии.
По результатам оценки вторичных критериев эффективности по окончании 6 недель терапии были получены статистически значимые различия среднего изменения в баллах по субшкалам «невнимательность» (-26 %), «гиперактивность/импульсивность» (-33 %), по индексу Коннерса (-33 %) шкалы SNAP-IV (рис. 3), среднего изменения значения по шкале ADHD Rating Scale IV (-30 %) (рис. 4), оценки по CGI-ADHD-S (52 % пациентов с СДВГ перешли в группу легкого течения), оценки по шкале CGI-I (улучшение или значительное улучшение было отмечено у 52 % пациентов) (рис. 5).
Изменение в % медианы баллов по субшкале “невнимательность”, SNAP-IV через 6 недель терапии
Вторичный критерий эффективности
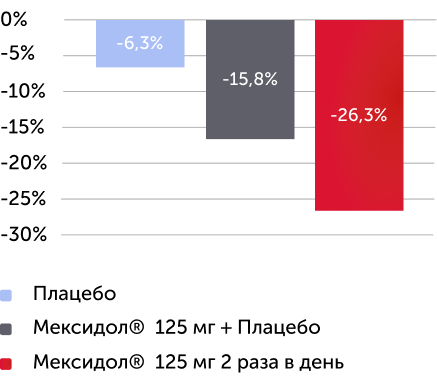
Различия между группами по субшкале “невнимательность” статистически значимы (р < 0,0001)
В группе Мексидол® 125 мг 2 раза в день отмечался более 26% регресс по субшкале “невнимательность”
Изменение в % медианы баллов по субшкале, SNAP-IV - “гиперактивность/импульсивность” через 6 недель терапии
Вторичный критерий эффективности
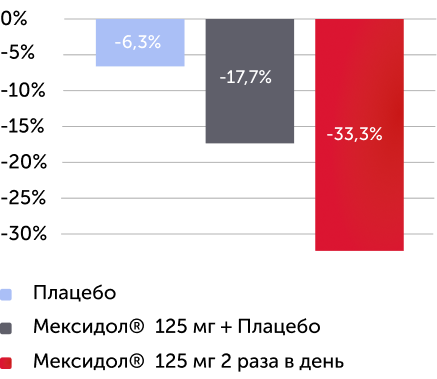
Различия между группами по субшкале “гиперактивность/импульсивность” статистически значимы ( р < 0,0001 )
В группе Мексидол® 125 мг 2 раза в день отмечался более 30% регресс по субшкале “гиперактивность/импульсивность”
Среднее изменение балла по субшкале SNAP-IV - индекс Коннерса через 6 недель терапии
Вторичный критерий эффективности
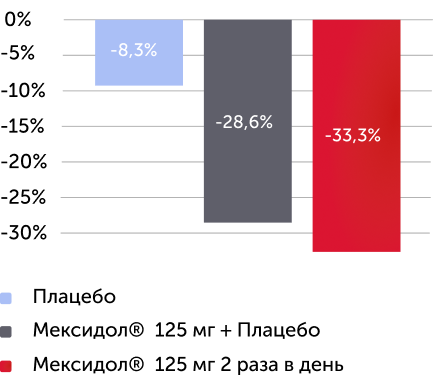
Различия между группами по индексу Коннерса статистически значимы ( р < 0,0001 )
В группе Мексидол® 125 мг 2 раза в день отмечался более 30% регресс по индексу Коннерса
Рисунок 3. Изменение среднего балла по субшкалам «невнимательность», «гиперактивность/импульсивность» и по индексу Коннерса через 6 недель терапии
Изменение в % медианы баллов по шкале оценки симптомов
СДВГ (ADHD Rating Scale - IV) через 6 недель терапии
Вторичный критерий эффективности
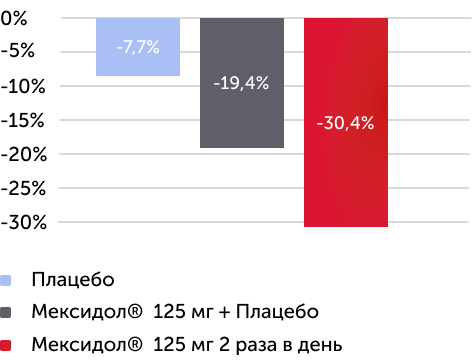
Различия между группами по шкале ADHD RS-IV статистически значимы (р < 0,0001)
В группе Мексидол 125 мг 2 раза в день отмечался более 30% регресс по шкале ADHD
Рисунок 4. Изменения среднего балла по шкале ADHD Rating Scale IV через 6 недель терапии
Шкала CGI-I
Шкала общего клинического впечатления - улучшение The Clinical Global Impression Scale - Improvement
Вторичный критерий эффективности
Изменения среднего балла по шкале ADHD Rating Scale IV через 6 недель терапии
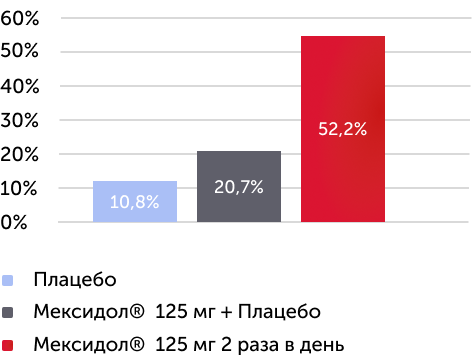
По окончании терапии между группами были выявлены статистически значимые различия по шкале общего клинического впечатления - улучшение ( р < 0,0001 )
По шкале CGI-I в группе Мексидол® 125 мг 2 раза в день более, чем у половины пациентов отмечалось улучшение или значительное улучшение
Рисунок 5. Динамика изменений по шкале CGI-I через 6 недель терапии
Безопасность
Результаты статистического анализа частоты возникновения нежелательных явлений, показателей лабораторных анализов и физикального обследования продемонстрировали отсутствие значимых различий между сравниваемыми группами по основным показателям безопасности. Таким образом, доказан сопоставимый характер профилей безопасности исследуемых режимов дозирования препарата Мексидол® и плацебо.
Выводы
Проведенное исследование показало, что препарат Мексидол® имеет высокую эффективность при лечении СДВГ у детей от 6 до 12 лет и безопасность, сопоставимую с плацебо. При оценке основного и большинства вторичных критериев эффективности схема лечения Мексидол® таблетки, покрытые пленочной оболочкой, 125 мг 2 раза в день показала свое преимущество перед схемой Мексидол® таблетки, покрытые пленочной оболочкой, 125 мг 1 раз в день.
Список литературы
- Заваденко Н.Н., Суворинова Н.Ю., Батышева Т.Т. и др. Результаты многоцентрового двойного слепого рандомизированного плацебоконтролируемого клинического исследования по оценке эффективности и безопасности препарата Мексидол в лечении синдрома дефицита внимания с гиперактивностью у детей (МЕГА). Журнал неврологии и психиатрии им. С.С. Корсакова. 2022;122(4):75‑86.
- Заваденко Н.Н., Суворинова Н.Ю., Заваденко А.Н. Возможности применения Мексидола в нейропедиатрии. Журнал неврологии и психиатрии им. С.С. Корсакова. 2023;123(9 вып. 2):43–50.
- Swanson JM, Schuck S, Porter MM, et al. Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales. Int J Educ Psychol Assess. 2012;10:51-70.
- Busner J, Targum SD. The Clinical Global Impressions Scale. Psychiatry Edgmont. 2007;4:28-37.
- Spence SH, Barrett PM, Turner CM. Psychometric properties of the Spence Children’s Anxiety Scale with young adolescents. J Anxiety Disord. 2003;17:605-625.
- Orgilés M, Fernández-Martínez I, Guillén-Riquelme A, Espada JP, Essau CA. A systematic review of the factor structure and reliability of the Spence Children’s Anxiety Scale. J Affect Disord. 2016;190:333-340.
- Заваденко Н.Н., Суворинова Н.Ю., Батышева Т.Т. и др. Результаты многоцентрового двойного слепого рандомизированного плацебоконтролируемого клинического исследования по оценке эффективности и безопасности препарата Мексидол в лечении синдрома дефицита внимания с гиперактивностью у детей (МЕГА). Журнал неврологии и психиатрии им. С.С. Корсакова. 2022;122(4):75‑86.
- Заваденко Н.Н., Суворинова Н.Ю., Заваденко А.Н. Возможности применения Мексидола в нейропедиатрии. Журнал неврологии и психиатрии им. С.С. Корсакова. 2023;123(9 вып. 2):43–50.
- Swanson JM, Schuck S, Porter MM, et al. Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales. Int J Educ Psychol Assess. 2012;10:51-70.
- Busner J, Targum SD. The Clinical Global Impressions Scale. Psychiatry Edgmont. 2007;4:28-37.
- Spence SH, Barrett PM, Turner CM. Psychometric properties of the Spence Children’s Anxiety Scale with young adolescents. J Anxiety Disord. 2003;17:605-625.
- Orgilés M, Fernández-Martínez I, Guillén-Riquelme A, Espada JP, Essau CA. A systematic review of the factor structure and reliability of the Spence Children’s Anxiety Scale. J Affect Disord. 2016;190:333-340.
ИНФОРМАЦИЯ ПРЕДНАЗНАЧЕНА ДЛЯ МЕДИЦИНСКИХ И ФАРМАЦЕВТИЧЕСКИХ РАБОТНИКОВ. ДАННАЯ ИНФОРМАЦИЯ НЕ МОЖЕТ СЛУЖИТЬ ЗАМЕНОЙ КОНСУЛЬТАЦИИ ВРАЧА.
Источник фото и изображений shutterstock.com