- A multicenter study - a study that was conducted in a large number of specialized clinical centers;
- Randomized - a study in which patients are distributed into groups randomly;
- Double blind placbo-controlled-a study in which neither a research doctor nor the patient know what the patient receives: the study drug or placebo.
Explification of Epica
with a Half -Hasmic Ischemic Stroke
Mexidol® showed a high degree of efficiency and safety with prolonged sequential therapy in patients in acute and early recovery periods of hemispherical ischemic stroke in a randomized double, blind multicenter-controlled in parallel groups of the epic study, which was met according to GCP standards (GOOD CLINICAL PRACTICE Clinical practice) [1].

The purpose of the study was to evaluate the effectiveness and safety of long -term
sequential therapy with Mexidol® according to the scheme:
10 days for 500 mg/day in/in drip, followed by a tablet (125 mg)
, 1 tablet 3 times a day for 8 weeks in patients with Holding
ischemic stroke in acute and early recovery periods.
- A multicenter study is a study that was conducted in a large number of specialized clinical centers.
- Randomized - a study in which patients are distributed into groups randomly.
- Double blind placbo-controlled-a study in which neither a research doctor nor the patient know what the patient receives: the study drug or placebo.

The purpose of the study was to evaluate the effectiveness and safety of long -term
sequential therapy with Mexidol® according to the scheme:
10 days for 500 mg/day in/in drip, followed by a tablet (125 mg)
, 1 tablet 3 times a day for 8 weeks in patients with Holding
ischemic stroke in acute and early recovery periods.
Research design
The study by Epica was attended by 151 patients (62 men and 89 women) aged 40 to 79 years with primary hemispherical ischemic stroke, confirmed by the methods of CT/MRI, hospitalized no later than 72 hours from the beginning of the development of stroke. 31 patients had diabetes (diabetes), 24 patients were carried out by thrombolytic therapy (TLT). All patients by simple randomization were distributed into two groups. The groups were comparable in numeral, demographic, clinical and laboratory data. Patients of the 1st group received consistent therapy: Mexidol® for 10 days for 500 mg/c/in drip, followed by tablets (125 mg), 1 tablet 3 times a day for 8 weeks, patients of the 2nd group received placebo according to a similar scheme. The duration of the course of treatment with Mexidol® was 66 (10+56) days.
Epica research design
The studied therapy included 2 consecutive stages and lasted - 66 days
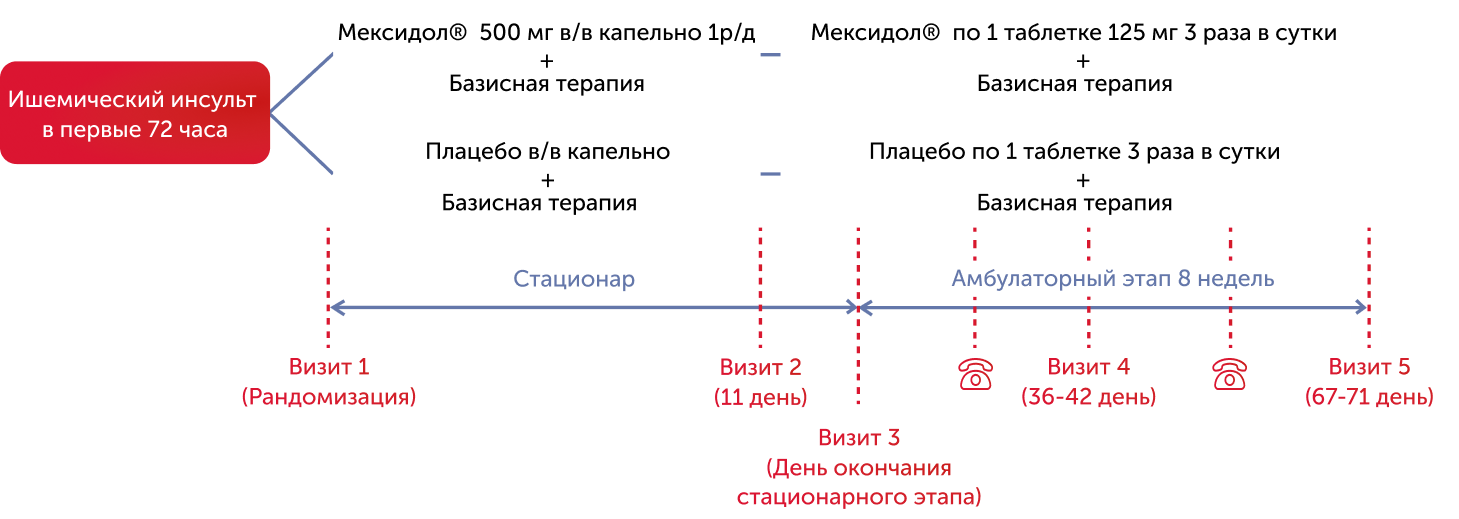
150 patients with ischemic strokes in 11 clinical centers of Russia received the studied therapy, the effectiveness analysis included 124 patients
The study scheme included 5 control stages of examination and two phone calls to observed patients:
- Visit 1 (1st day of research),
- Visit 2 (11th day, completion of the injection course),
- Visit 3 (end of the stationary stage of treatment),
- Visit 4 (36-42 Day of the study, completion of 1 month of tablet therapy),
- Visit 5 (67-71, completion of the entire course of treatment).
- Active phone calls to patients were produced between the 3rd and 4th, 4th and 5th visits.
Efficiency criteria
- The primary criterion for effectiveness included the test results on the Rankin modified scale at the time of the end of the therapy (MRS, Modified Rankin Scale), which allows you to evaluate the degree of vital impairment (disabilities), independence and outcome of rehabilitation.
- When analyzing the secondary criteria for effectiveness, the results of testing on the scales at the time of the end of the therapy were taken as a basis:
- NIHSS (The National Institutes of Health Stroke Scale - a stroke scale of the National Institute of Health), characterizes the severity of neurological deficiency
-Beck Depression Inventory (Bek depression scale-test-questionnaire of depression), characterizes the severity of depression
- EQ -5D (EuroQol Quality of Life Scale - questionnaire for assessing the quality of life). As well as a screening questionnaire for determining cognitive impairment and a set of tests for evaluating frontal dysfunction.
- The safety of treatment was evaluated according to the physics examination; general clinical, biochemical blood tests, coagulograms, general urine analysis, ECG, frequency and severity of unwanted phenomena.
Results
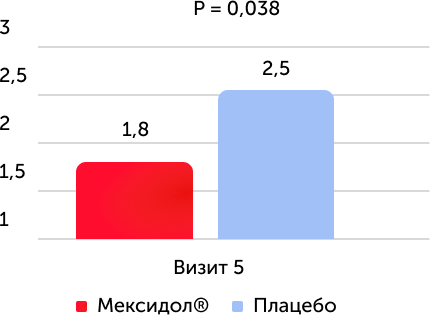
The average value of the point for MRS (all patients)
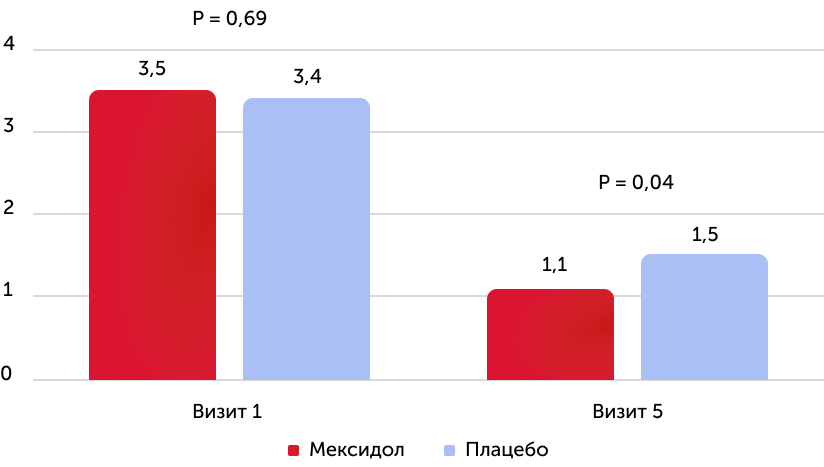
When analyzing the results according to the primary criterion of effectiveness, it was found that in the group of therapy with the drug Mexidol, a reliable improvement in vital functions and a decrease in the symptoms of the disease and functional disorders compared to placebo: at the time of the end of the therapy, the average score on the MRS was statistically significantly lower in the group of use of the drug Mexidol, It was also a reliably more pronounced dynamics of reducing the average score by MRS. The proportion of patients who have reached the recovery corresponding to 0–2 points by MRS to 5 visits was reliably higher in the Mexidol preparation group compared to placebo.
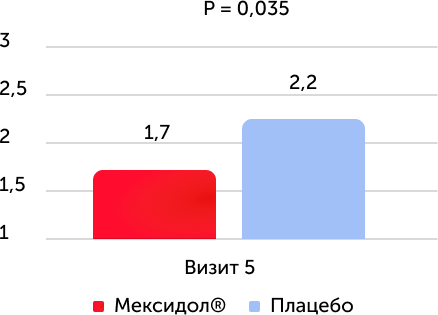
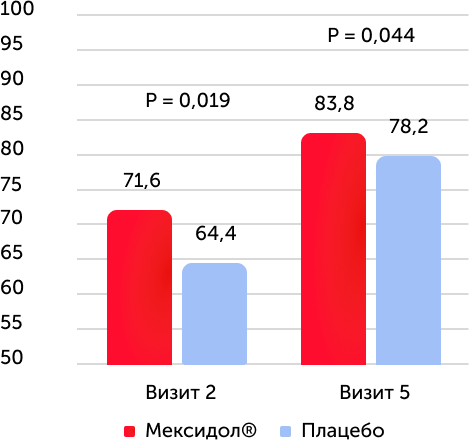
The average value of the point on the NIHSS scale at the end of therapy
The average value of the score on the NIHSS scale with diabetes mellitus
The study of the results of secondary effectiveness criteria showed that during testing on the NIHSS scale in both groups, positive dynamics were revealed. By the time the entire course of therapy is completed, the neurological deficiency was reliably lower in the therapy group with Mexidol®. When analyzing the category of patients with diabetes who received Mexidol® therapy, a decrease in neurological deficiency was more pronounced compared to the placebo group.
The whole population
Patients with TLT
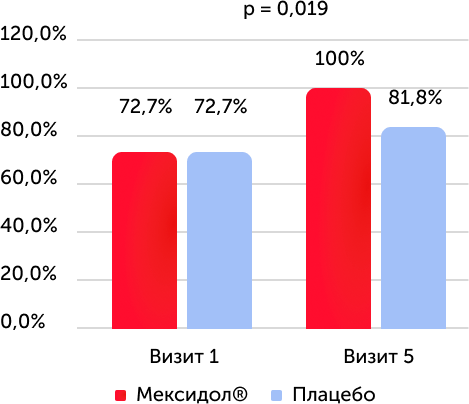
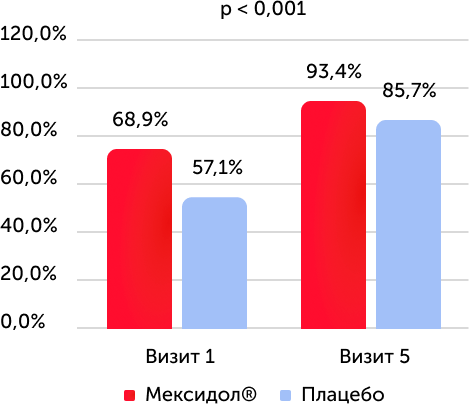
The results of testing on the Bek depression scale, the lack of symptoms of depression, %
When assessing the condition on the Bek depression scale, in both groups there was a statistically significant increase in the number of patients with the absence of symptoms of depression from 1 to 5 visits (p <0.001), a similar dependence was detected in patients with TLT in the 1st group (p (p. = 0.019). When assessing the state of the cognitive-affectionative subshparel, the Bek depression scale in both groups observed a statistically significant difference between the initial level and values at the time of the end of the therapy.
The whole population
Patients with diabetes mellitus
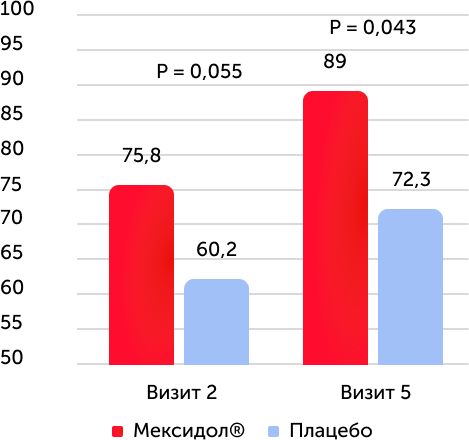
EQ-5D testing results, average value
When testing the EQ-5D quality questionnaire, a statistically significant dynamics was observed during the study, including the difference between values in the 1st and 5th visits in both groups. The results between groups are statistically significant in the 2nd and 5th visits. In subpopulation of patients with diabetes in the therapy group, the Mexidol® quality of life was reliably higher by the end of therapy.
EQ-5D testing results, mobility scale
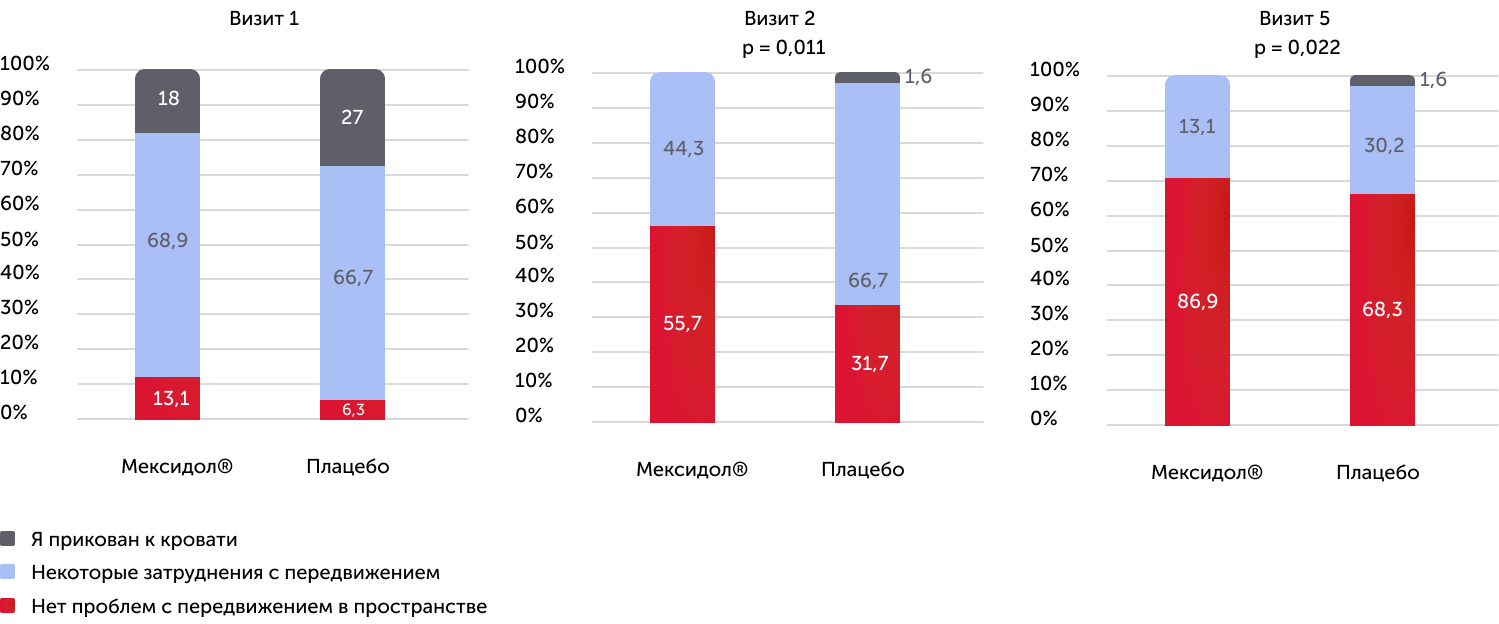
In a separate analysis of the functions according to the EQ-5D questionnaire on the health scale “Movement” in both groups of therapy, they revealed a statistically significant linear dependence in the direction of increasing the number of patients with lack of problems with movement, as well as a statistically significant difference between groups in 2 and 5- m visits. It was established that 87% of patients of the 1st group did not have problems related to movement; 79% of patients noted that they did not have problems with self -care; 71% of patients believed that they had no problems with the implementation of everyday affairs (work, study, household chores, family duties, leisure time); 85% of patients did not feel pain and discomfort; 89% did not feel anxiety and depression.
In the group of therapy with Mexidol®, the quality of life was reliably higher, already starting from the second visit.
Subanalis of the study of epic by age criterion,
including in patients with concomitant diabetes mellitus
In the process of Subanalysis of the study of Epic, the effectiveness and safety of Mexidol® in patients of different age groups in the acute and early recovery periods of the hemisphere ischemic stroke was studied [2].
- All patients were divided into subgroups by age:
- younger than 60 years,
- 60–75 years,
- 76–90 years.
The results on the MRS were evaluated at the end of the course of therapy, the Bartel index, the Bek depression scale, and the European questionnaire of quality assessment (EQ-5D).
The effectiveness of Mexidol® was demonstrated on all
scales used and did not differ in various age groups.
At the time of the end of the therapy, the average MRS score compared with the placebo was statistically significantly lower in patients of all age groups, including 76–90 years in the group. The greatest dynamics of reducing the average score by MRS by the end of therapy was observed in patients 60–75 years.
The difference in values on the MRS scale, before and after therapy
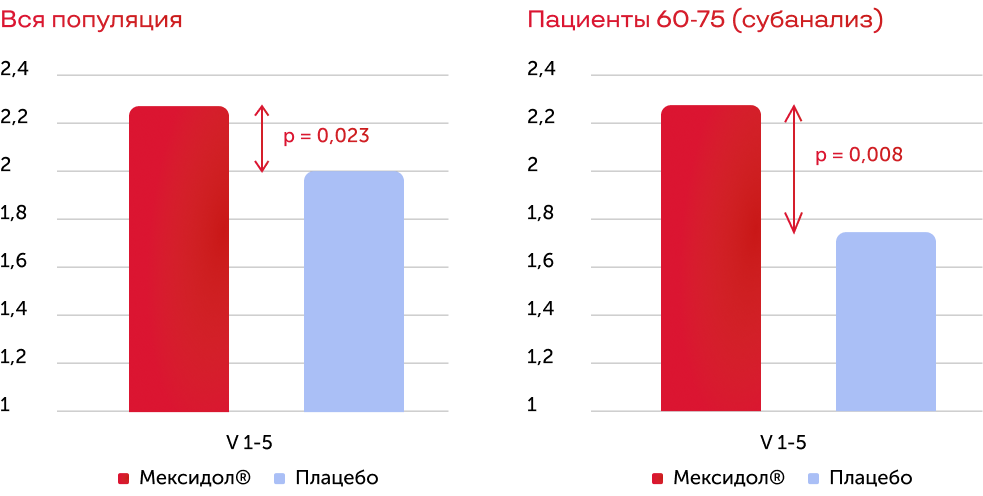
During cross analysis, it was shown that the same tendency is observed in patients with diabetes. In patients of 76–90 years, compared with placebo, the severity of cognitive-affective symptoms of depression was statistically significant, and the proportion of patients with lack of problems with the implementation of everyday affairs increased. Similar results were obtained in a subgroup of 60–75 years with diabetes. In patients with diabetes, compared to placebo, the level of everyday activity has also increased statistically significant and the quality of life has improved.
When studying the safety of reliable differences in the frequency of identification of undesirable phenomena in patients, all studied groups were not detected. The data obtained indicate a comparable security profile of the drug Mexidol® (a solution for iv and iv and in/m of administration and tablets covered with a shell) and placebo when used in patients in the acute and early recovery periods of a half -rushed ischemic stroke.
Thus, the use of
Mexidol® in the acute and early restoration periods of
ischemic stroke in all age groups,
including patients with diabetes, is recommended.
Conclusions
Thus, the use of consistent therapy with Mexidol® contributed to a reliable decrease in symptoms and functional disorders in patients with hemispherical ischemic stroke:
- During a long consistent therapy, Mexidol noted a reliably more pronounced improvement in life, appreciated by the MRS scale,
- The neurological deficit was reliably lower in the Mexidol therapy group when testing on the NIHSS scale at the end of therapy compared to placebo
- Mexidol therapy contributed to a reliable improvement in the quality of life (EQ-5D questionnaire) and a decrease in depression (Bek scale), starting from the second visit
The results of the study indicate that Mexidol® in
consecutive therapy has a favorable profile
of tolerance and safety. The use of Mexidol® is
important for the successful treatment of patients in the acute
and early restoration periods of ischemic stroke.
List of literature
- Stakhovskaya L.V., Shamalov N.A., Khasanova D.R., Melnikova E.V., Agafyina A.S., Golikov K.V., Bogdanov E.I., Yakupova A.A., Roshkovskaya L .V., Lukin L.V., Lokstanova T.M., Parinenova I.E., Shchepanevich L.A. The results of a randomized dual-blind multicenter placebo-controlled in parallel groups of the study and safety of Mexidol with prolonged consistent therapy in patients in the acute and early recovery periods of the hemisphere ischemic stroke (Epica). Journal of neurology and psychiatry named after S.S. Korsakova. Special letters. 2017; 117 (3-2): 55-65.
- Stakhovskaya L.V., Mkhitaryan E.A., Tkacheva O.N., Ostroumova T.M., Ostroumova O.D. The effectiveness and safety of Mexidol in patients of different age groups in the acute and early recovery periods of hemispherical ischemic stroke (the results of subanarized a randomized double blind multicenter placebo-controlled in parallel groups of the epic study). Journal of neurology and psychiatry named after S.S. Korsakova. Special letters. 2020; 120 (8): 49–57.
THE INFORMATION IS INTENDED FOR HEALTHCARE AND PHARMACEUTICAL PROFESSIONALS. THIS INFORMATION IS NOT INTENDED AS A SUBSTITUTE FOR MEDICAL ADVICE.
Source of photos and images Shutterstock.com